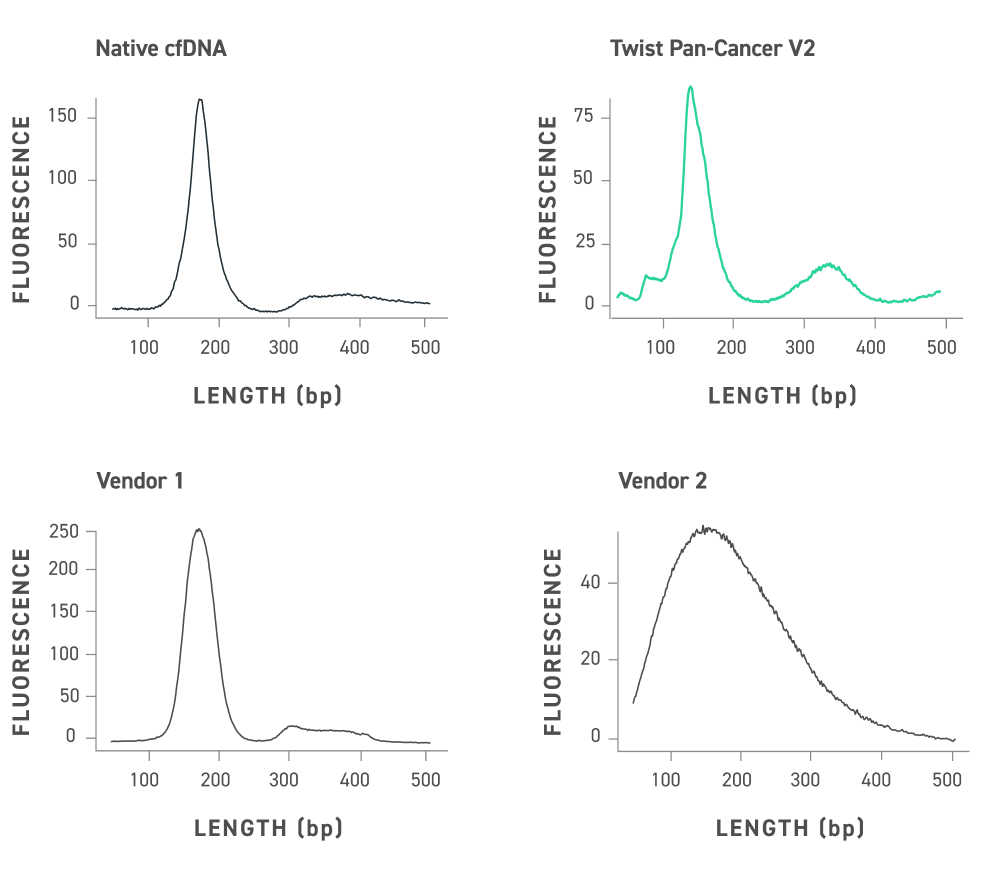
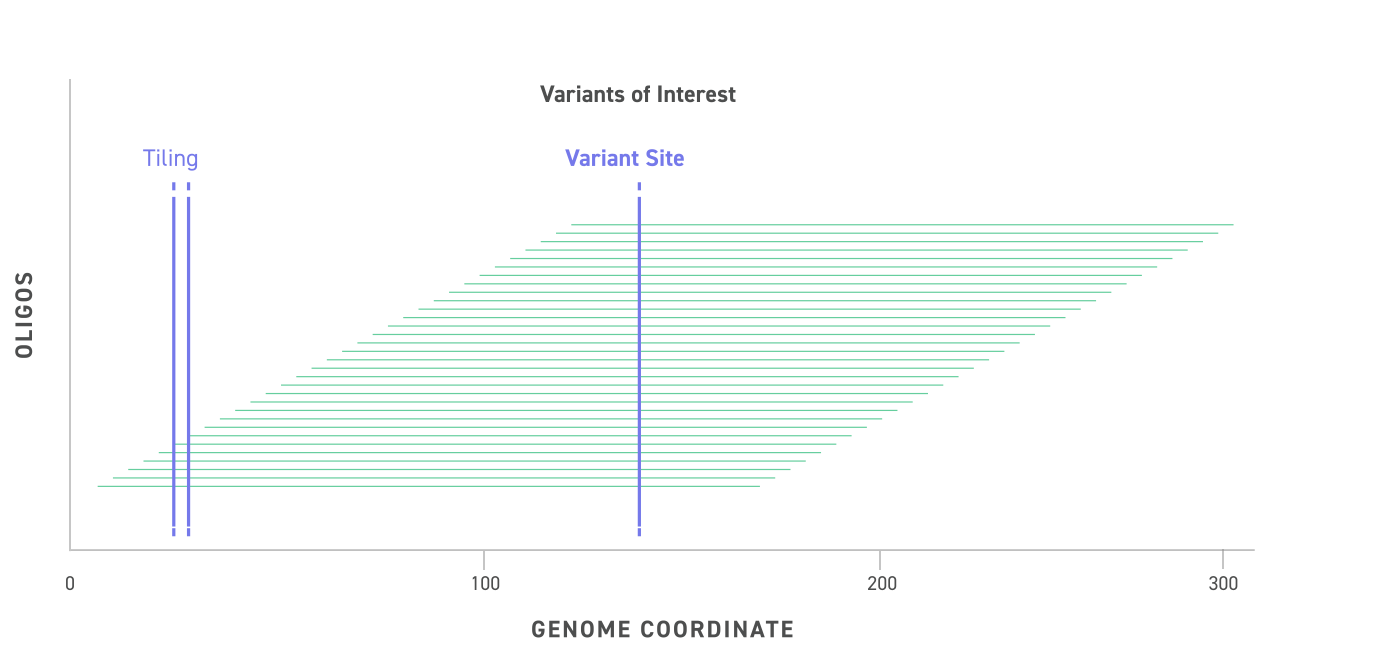
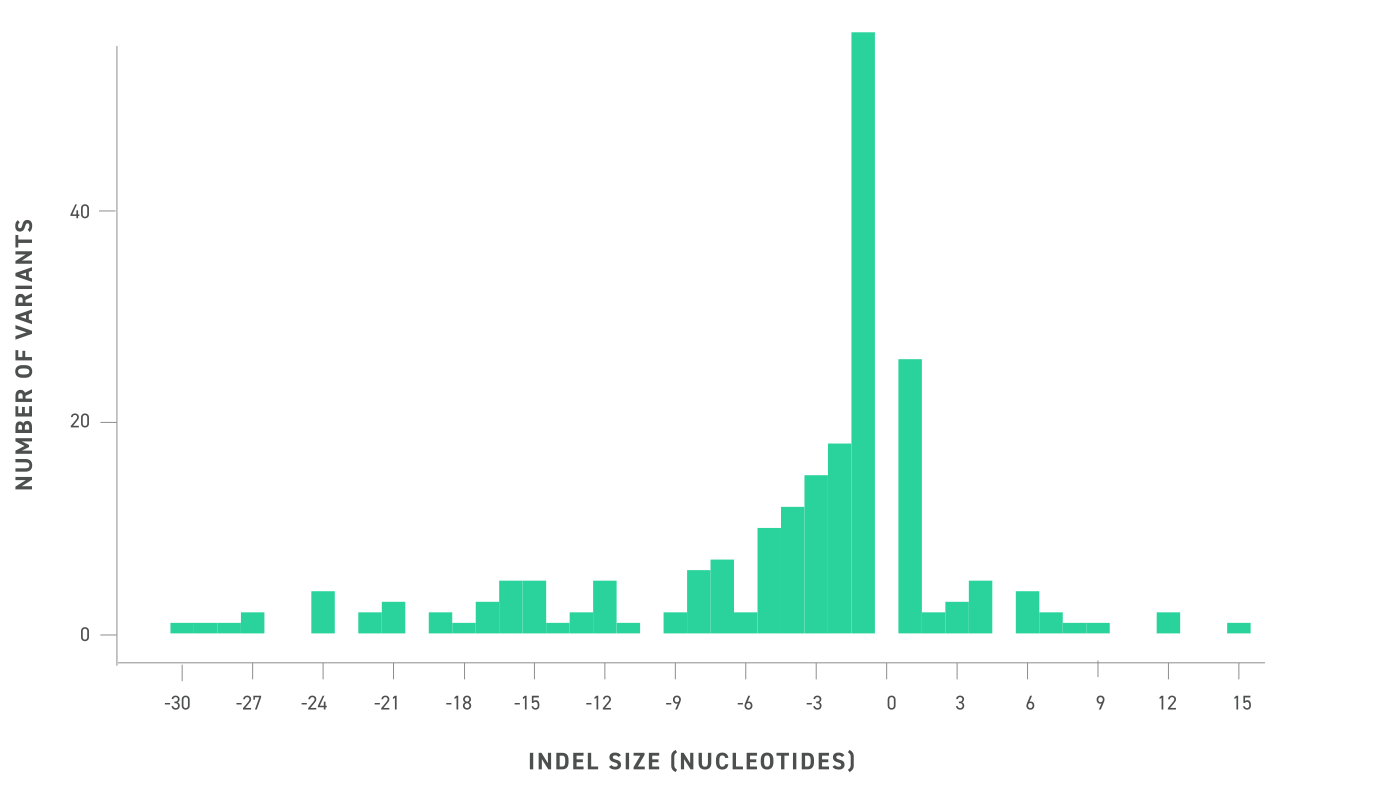
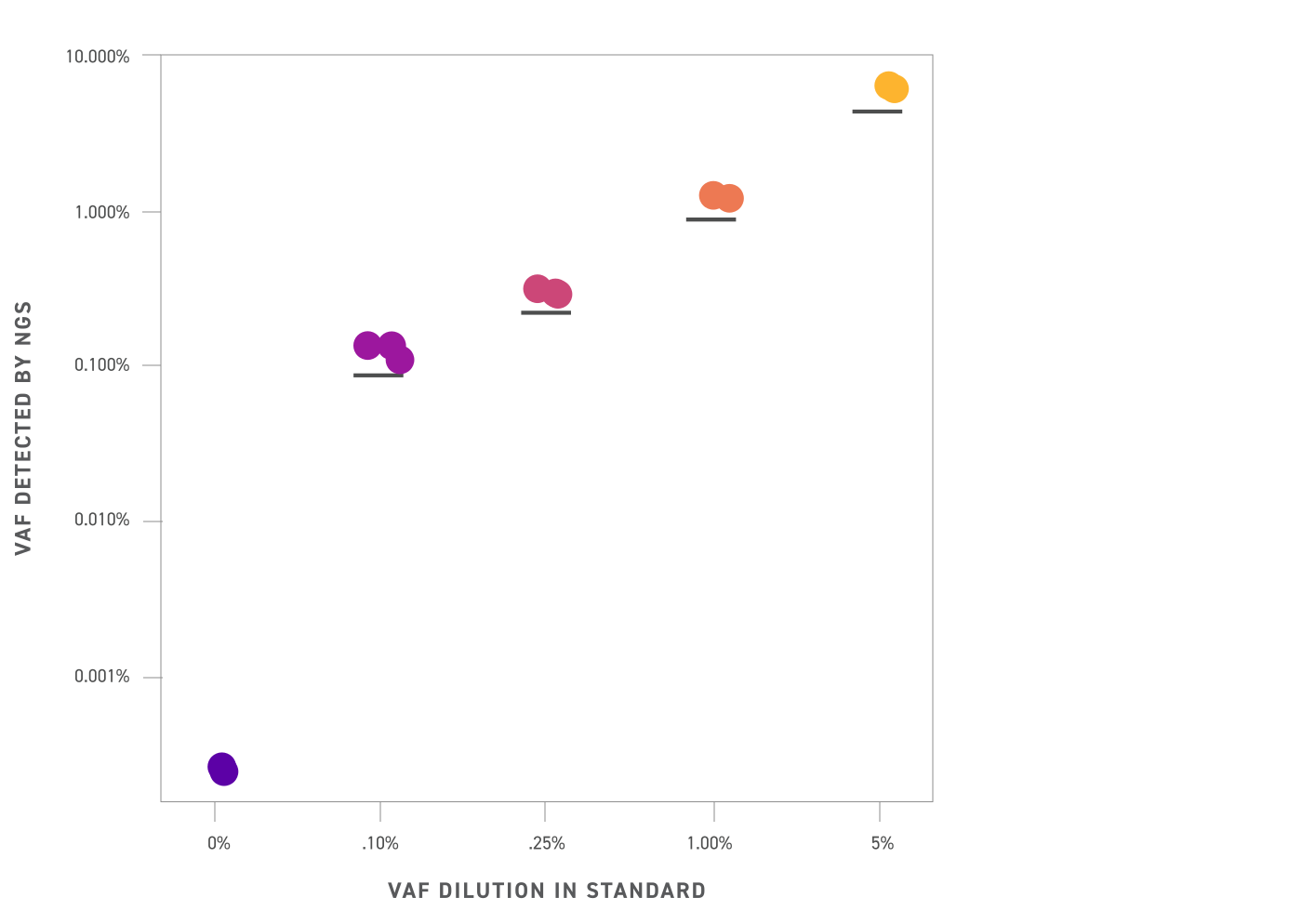
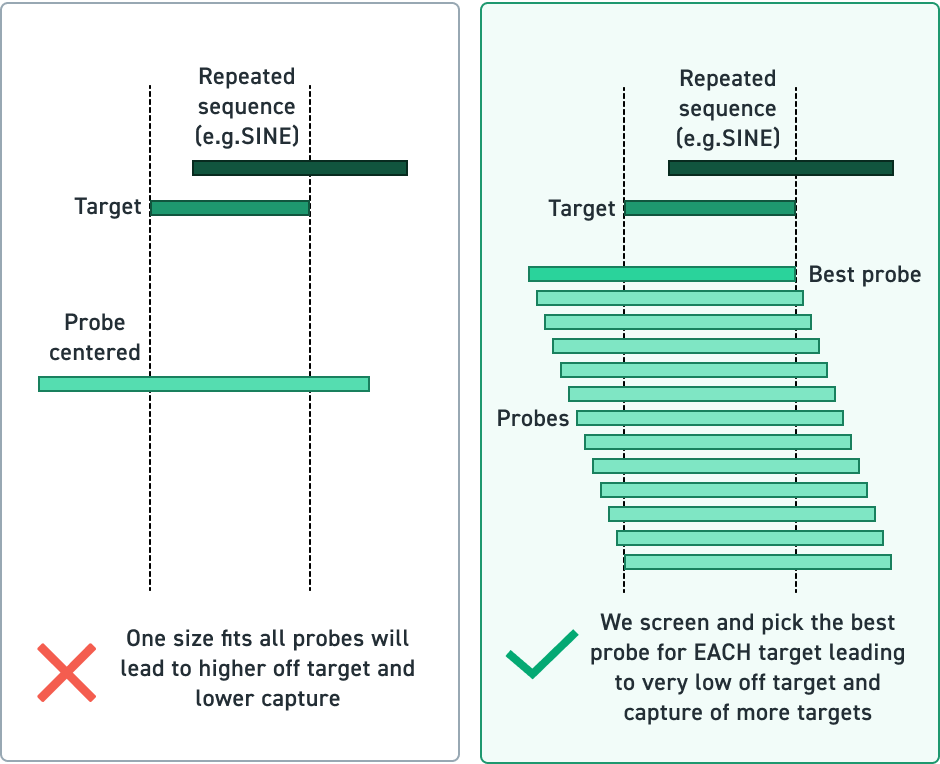
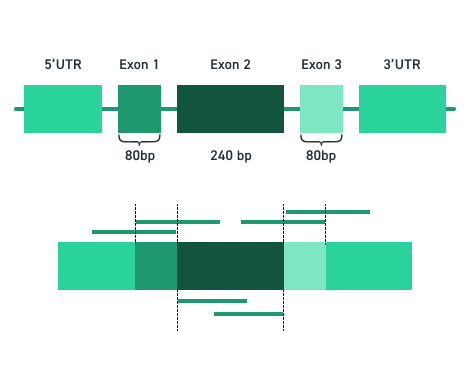
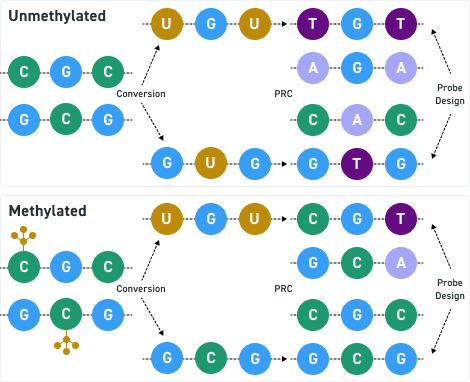
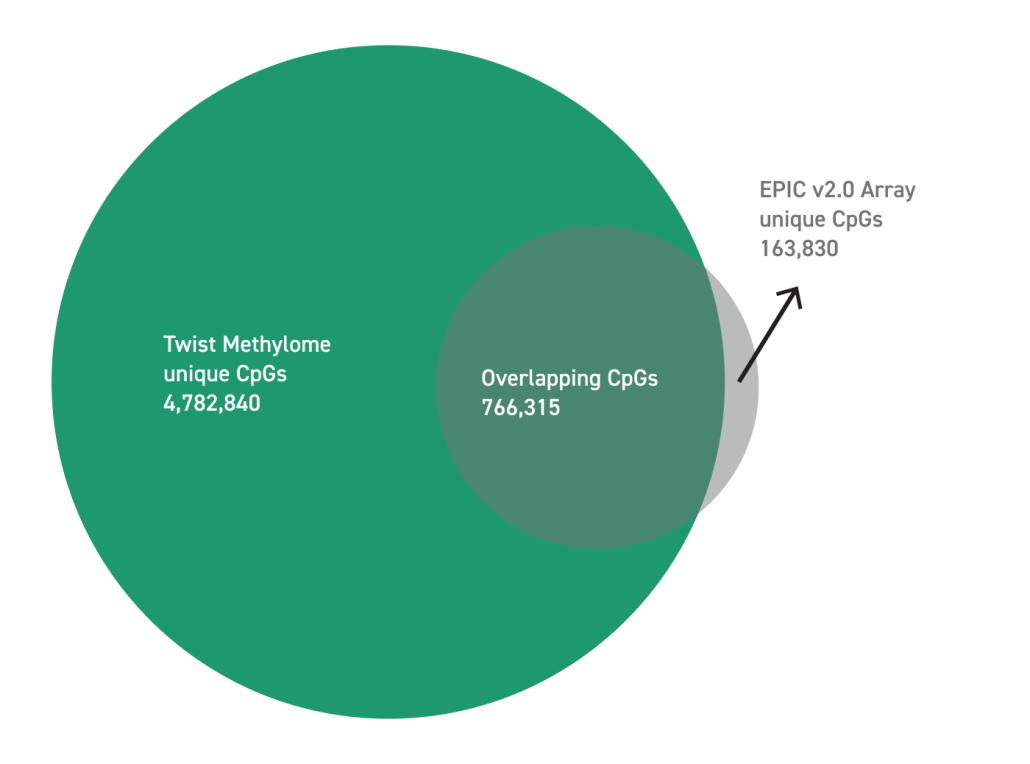
The Twist cfDNA Pan-Cancer Reference Standard v2 is a meticulously engineered tool tailored for genomics professionals developing and validating NGS-based liquid biopsy assays. This reference standard provides a reliable and reproducible resource for detecting clinically relevant variants, enabling researchers to confidently evaluate assay performance at every stage. By integrating a wild-type (WT) cell-free DNA (cfDNA) background with synthetic oligonucleotides carrying mutant alleles, the material delivers exceptional precision and sensitivity.
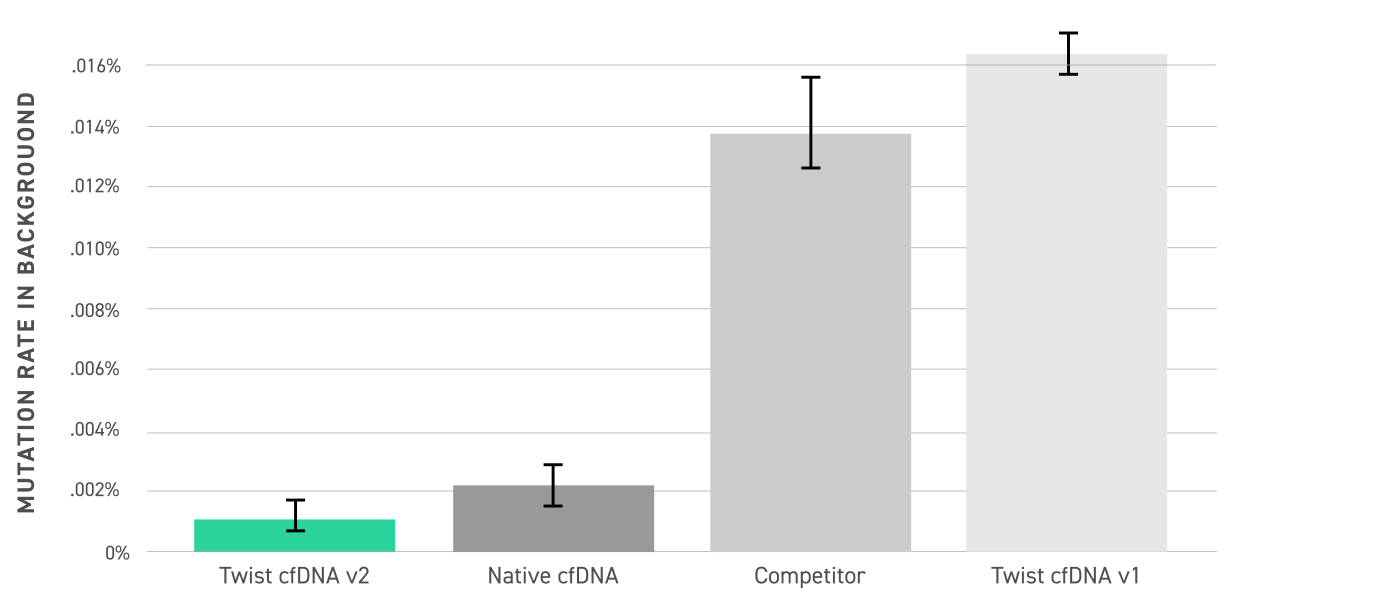
Researchers can leverage this standard to define critical analytical parameters, including the Limit of Detection (LoD) and Limit of Blank (LoB), ensuring robust assay calibration and quality control. Ideal for both early-stage assay development and ongoing process monitoring, the Twist cfDNA Pan-Cancer Reference Standard v2 empowers genomics teams to accelerate liquid biopsy innovation while maintaining rigorous accuracy and reproducibility.