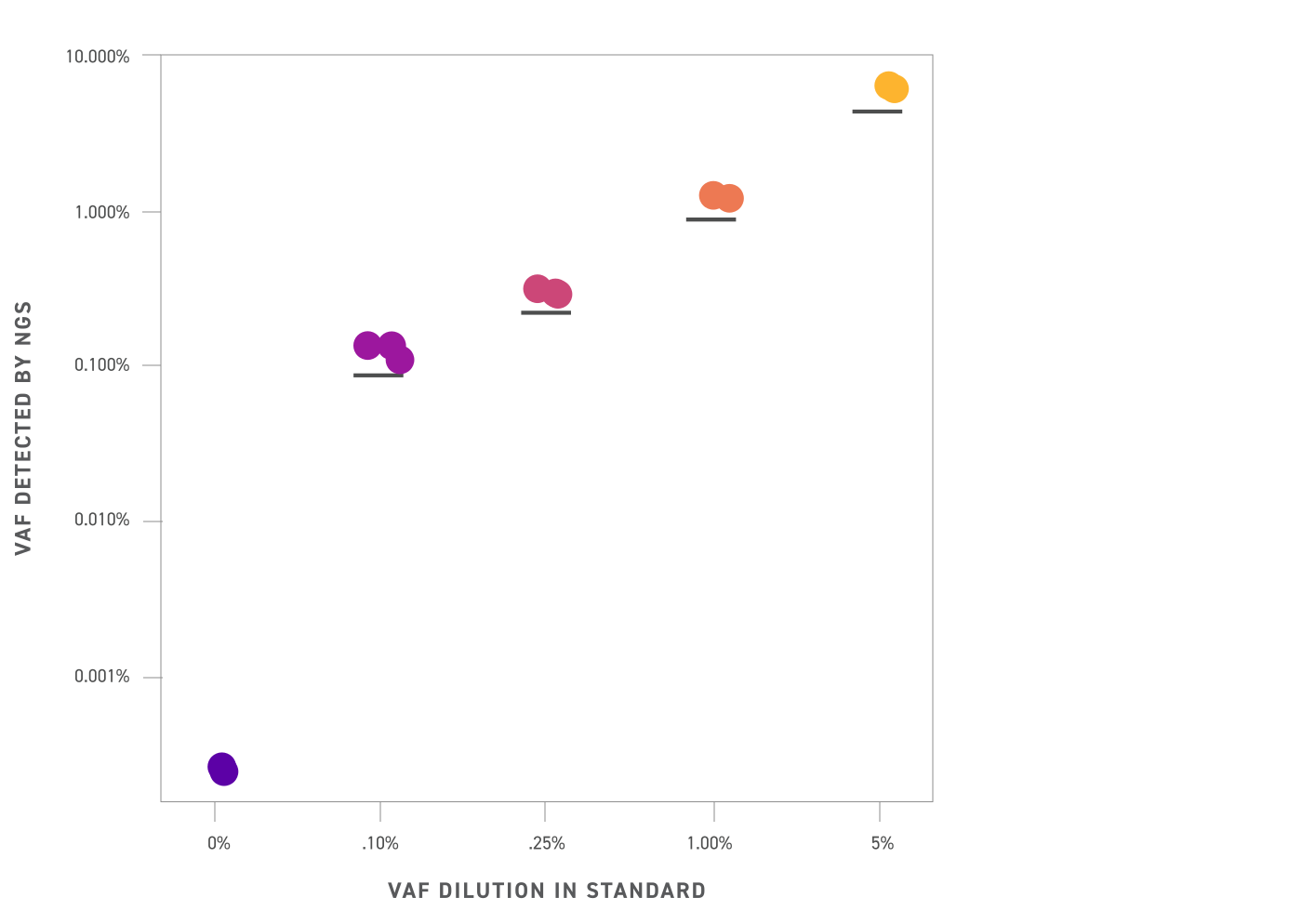
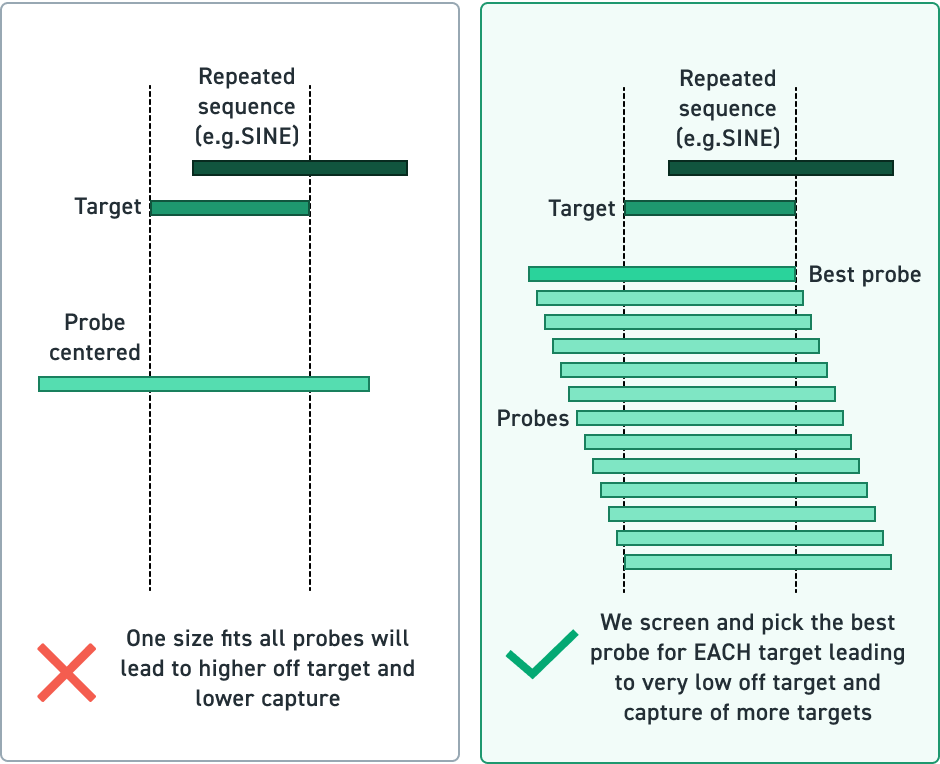
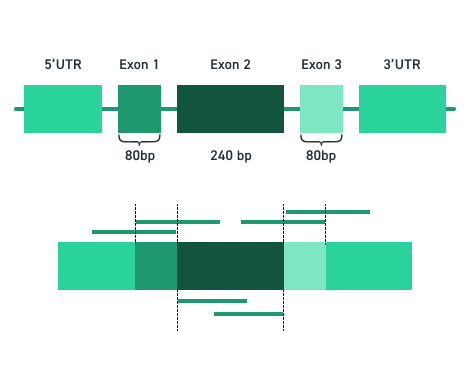
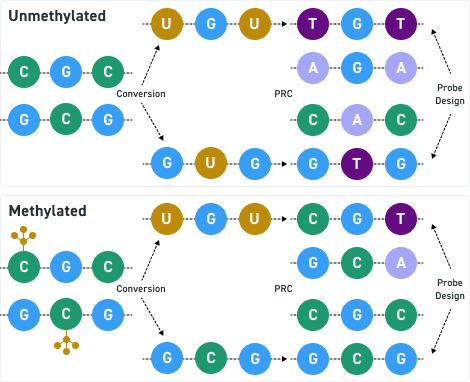
DNA Cartridges
/in BiOptic, Consumables, Partners/by Harshita SharmaPRODUCTS
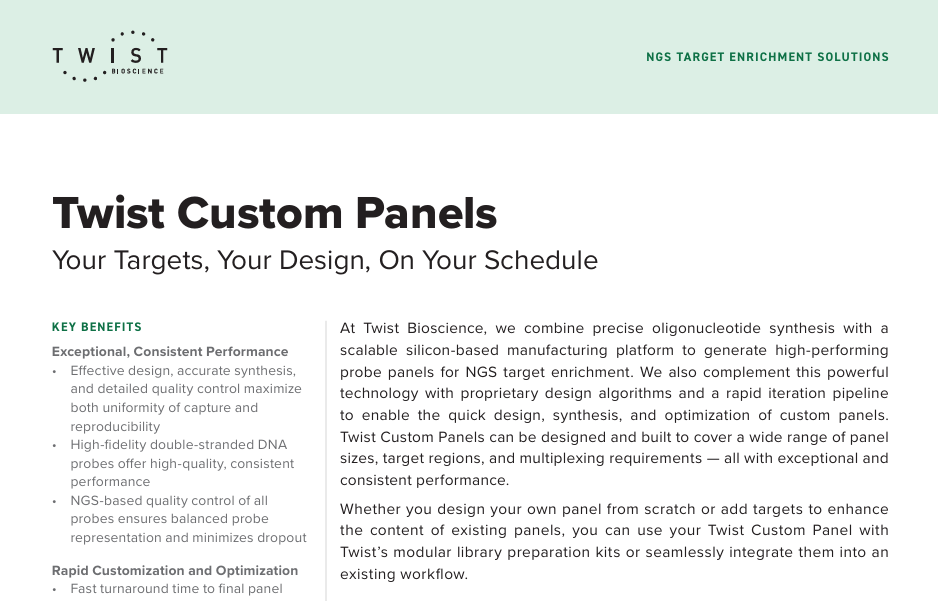
DNA Cartridges
DNA Cartridges
S2 Standard Cartridge Kit
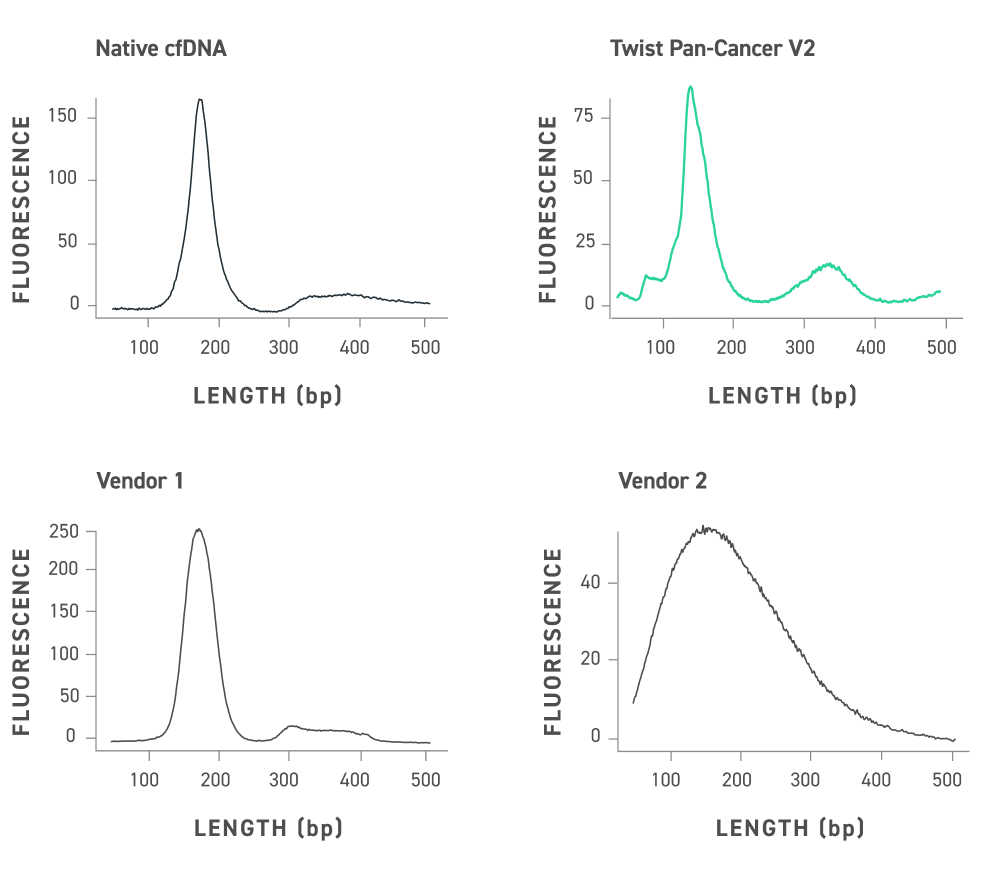
- S2 cartridge is for PCR products, small-sized DNA, and NGS quality control (Fragmentation and Library sample) analyses.
- The analysis time is 2~3 minutes and the best resolution is 4~10bp if the sample size is less than 500bp.
- It has a recommended size range up to around 5,000bp and a detection limit of about 0.1 ng/μl in low concentration.
- Each cartridge can analyze 200 runs for single-channel cartridge or 800 runs for 4-channel cartridge.
- The cartridge can be stored at 4~27°C and is valid for 6 months.

S1 High Resolution Cartridge Kit
- The analysis time is 2~5 minutes and the best resolution is 1~4bp if the sample size is less than 500bp.
- It provides high resolution result with multiple methods from low to high voltage (2~10kV) for user to choose from.
- It has a recommended analysis range up to around 5,000bp and a detection limit of about 0.1 ng/μl in low concentration.
- Each cartridge can analyze 200 runs for single-channel cartridge or 800 runs for 4-channel cartridge.
- The cartridge can be stored at 4~27°C and is valid for 6 months.
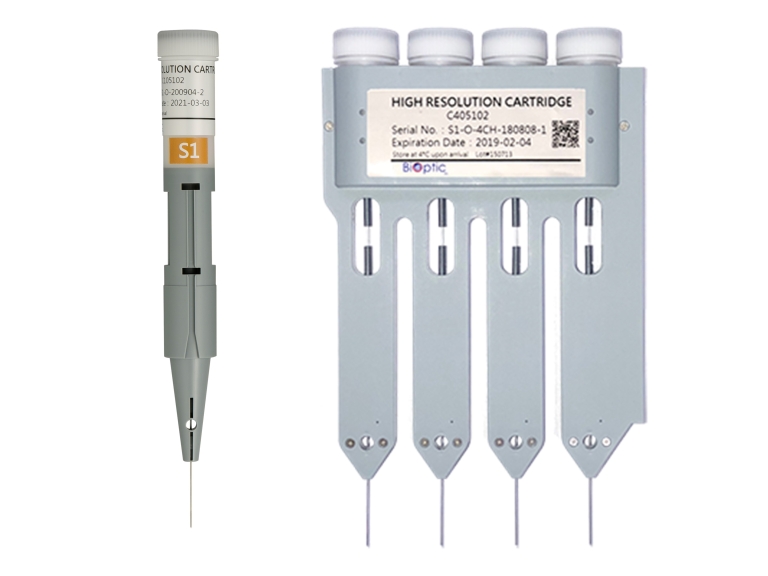
S3 Kilobase Cartridge Kit
- S3 cartridge is for large-sized DNA analysis.
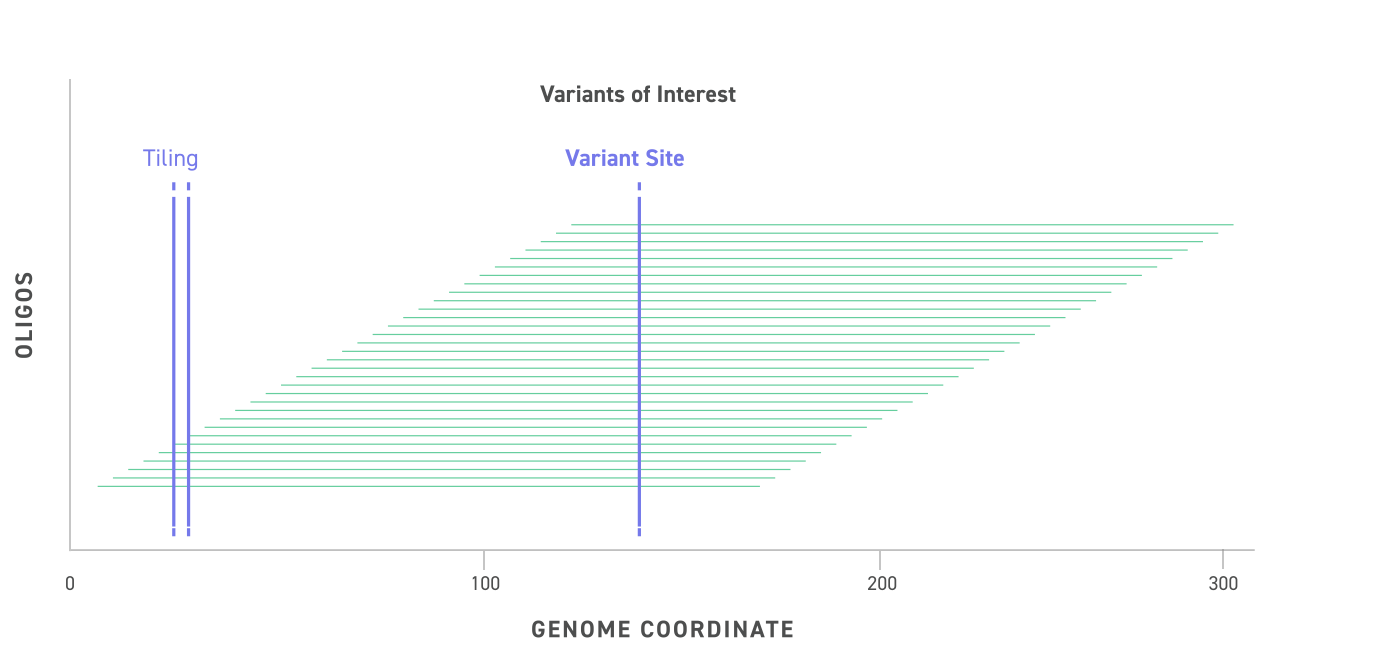
- It has a recommended 5~165kb size range and is ideal for quality control of second and third generation sequencing.
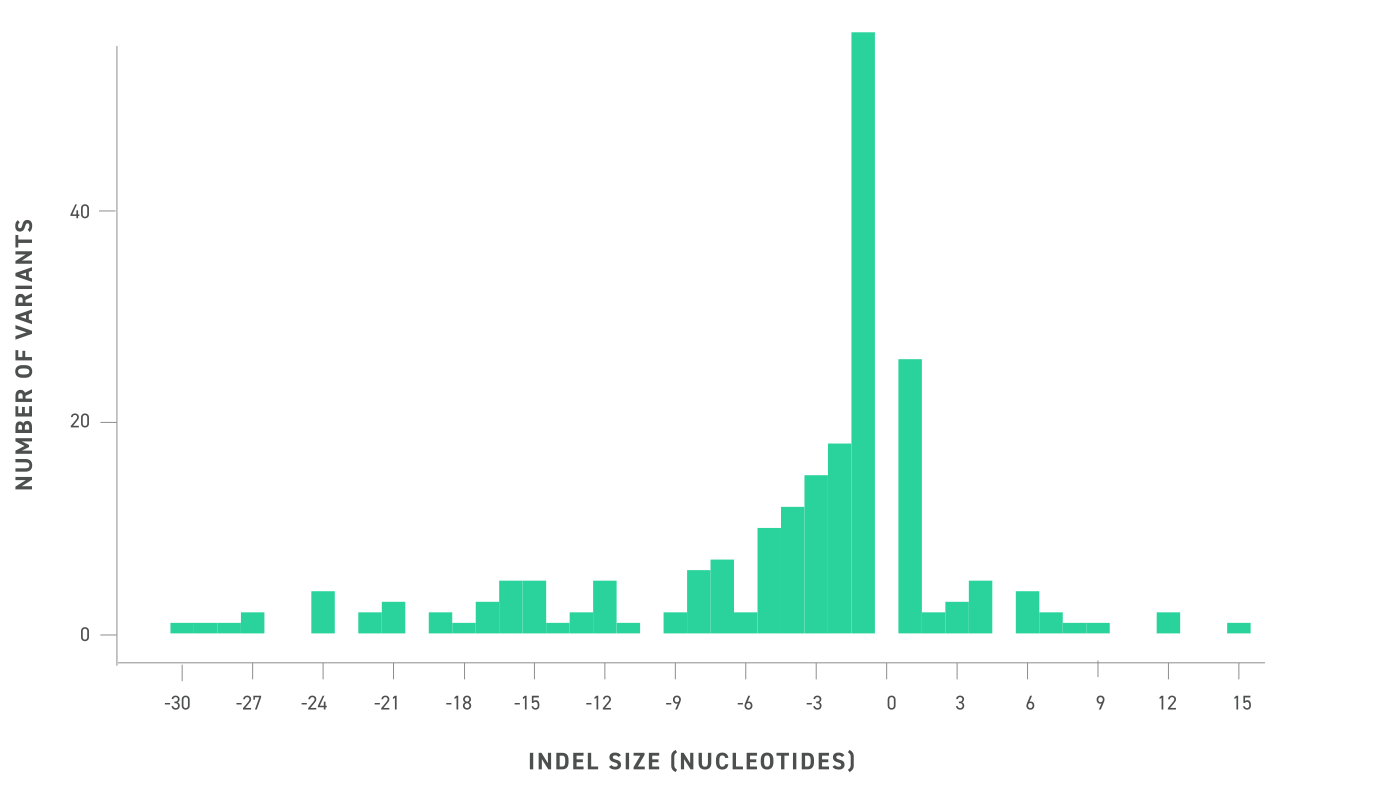
- The system provides DNA Quality Number (DQN) for gDNA integrity reference.
- Each cartridge can analyze 200 runs for single-channel cartridge or 800 runs for 4-channel cartridge.
- The cartridge can be stored at 4~27°C and is valid for 6 months.

F3 Fast Cartridge Kit
- The analysis time is 90 seconds and can be applied to PCR product analysis.
- It has a recommended analysis range up to around 5,000bp for DNA.
- It runs on Qsep100 and Qsep400 only.
- Each cartridge can analyze 300 runs for single-channel cartridge or 1200 runs for 4-channel cartridge.
- The cartridge can be stored at 4~27°C and is valid for 4 months.

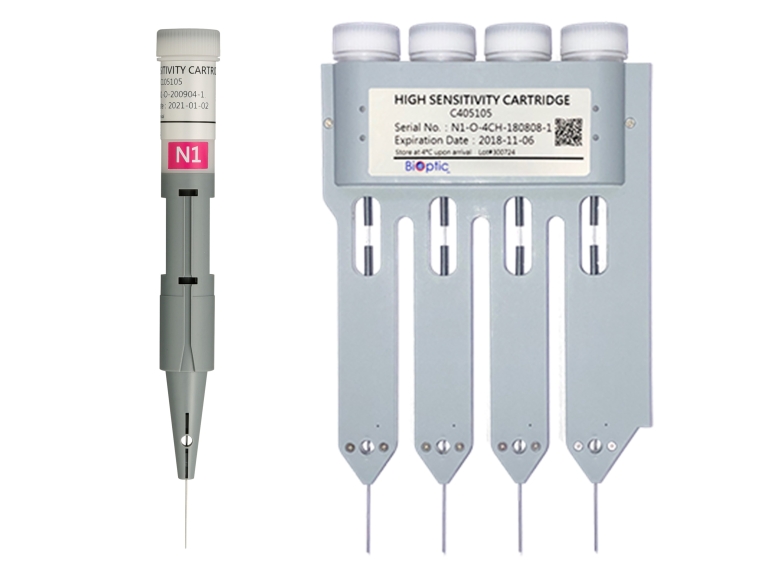
N1 High Sensitivity Cartridge Kit
- The sample concentration for analysis has a detection limit of about 5 pg/μl (or 1 pg/μl if dissolved in ddH2O) and the analysis time is about 3 minutes.
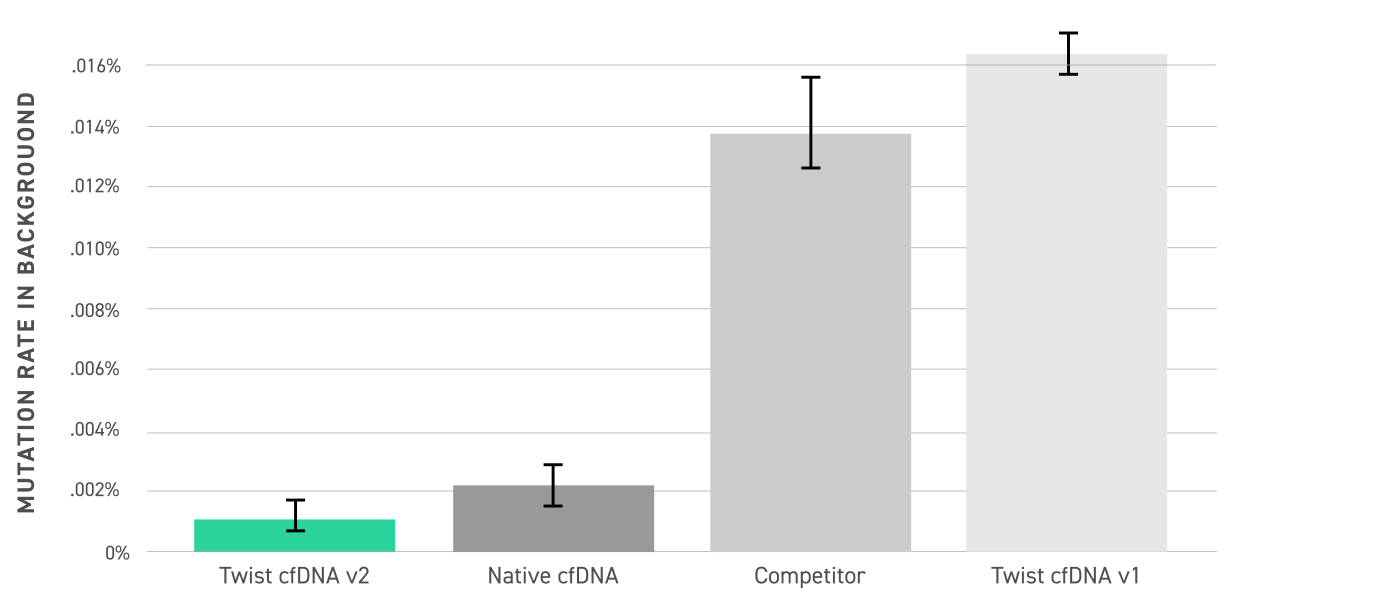
- It can be used for analysis of low concentration DNA fragment and quality control of cell-free DNA.
- It has a recommended analysis range up to around 5,000bp for DNA.
- Each cartridge can analyze 100 runs for single-channel cartridge or 400 runs for 4-channel cartridge.
- The cartridge can be stored at 4~27°C and is valid for 4 months.
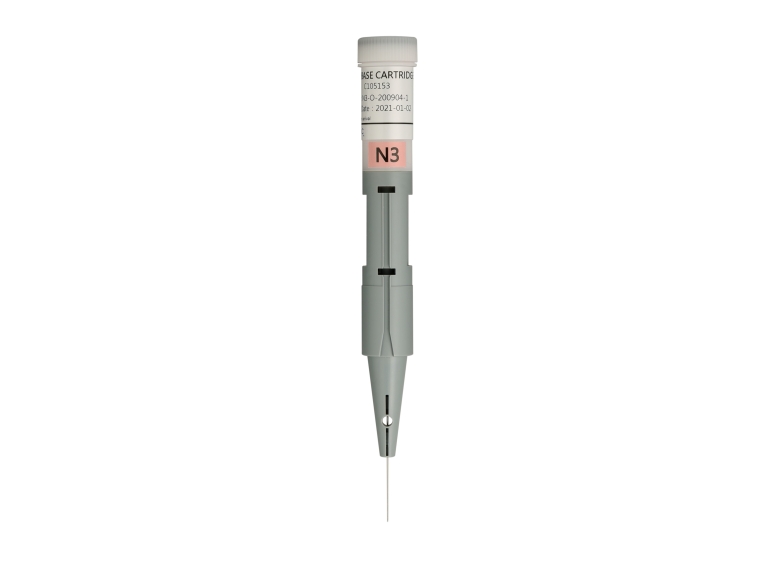
N3 High Sensitivity Kilobase Cartridge Kit
- N3 cartridge is for low concentration large-sized DNA analysis.
- It has a recommended 5~165kb size range and is ideal for quality control of second and third generation sequencing.
- The system provides DNA Quality Number (DQN) for gDNA integrity reference.
- Each cartridge can analyze 200 runs for single-channel cartridge or 800 runs for 4-channel cartridge.
- The cartridge can be stored at 4~27°C and is valid for 4 months.
Ready To Order?
Our team can help you in placing the order. Click below to get a quote and fast ordering.
BiOptic Consumables Portfolio
Have a question?
Get a call from your local Decode Science representative to help you find the best fit genomics products for you.
Or give us a call at: