Huber, H., Montoliu-Gaya, L., Brum, W.S. et al. A minimally invasive dried blood spot biomarker test for the detection of Alzheimer’s disease pathology. Nat Med (2026). https://doi.org/10.1038/s41591-025-04080-0
The Simoa Dry Blood Extraction Kit is a validated, device-agnostic solution for extracting analytes from dried plasma and dried blood spot (DPS/DBS) samples for use with Simoa® assays. It enables consistent, reproducible recovery of low-abundance biomarkers while maintaining the femtogram-level sensitivity required for clinical and translational research.
Designed to support decentralized and remote sample collection, the kit simplifies pre-analytical workflows without compromising data quality. It is well suited for longitudinal studies, multi-site trials, and settings where traditional venous sampling and cold-chain logistics are impractical.
Validated across multiple dried plasma and dried blood spot collection devices, enabling consistent analyte recovery independent of collection format. This supports reliable data generation across decentralized, remote, and longitudinal study designs.
A harmonized extraction protocol controls critical pre-analytical variables, including elution conditions, centrifugation parameters, and buffer composition. This reduces operator- and site-dependent variability and improves reproducibility across batches and study sites.
The extraction process is optimized to maintain the ultra-high sensitivity of Simoa® assays, enabling reliable detection of low-abundance biomarkers such as p-Tau 217. Suitable for neurological, inflammatory, and systemic biomarker applications where signal integrity is critical.
Designed specifically for downstream use with Simoa® kits, the workflow integrates into existing laboratory processes without the need for additional assay optimization or protocol development.

Hanna Huber
Nutrition Scientist // Ph.D // Postdoctoral researcher @DZNE Bonn & University of Gothenburg
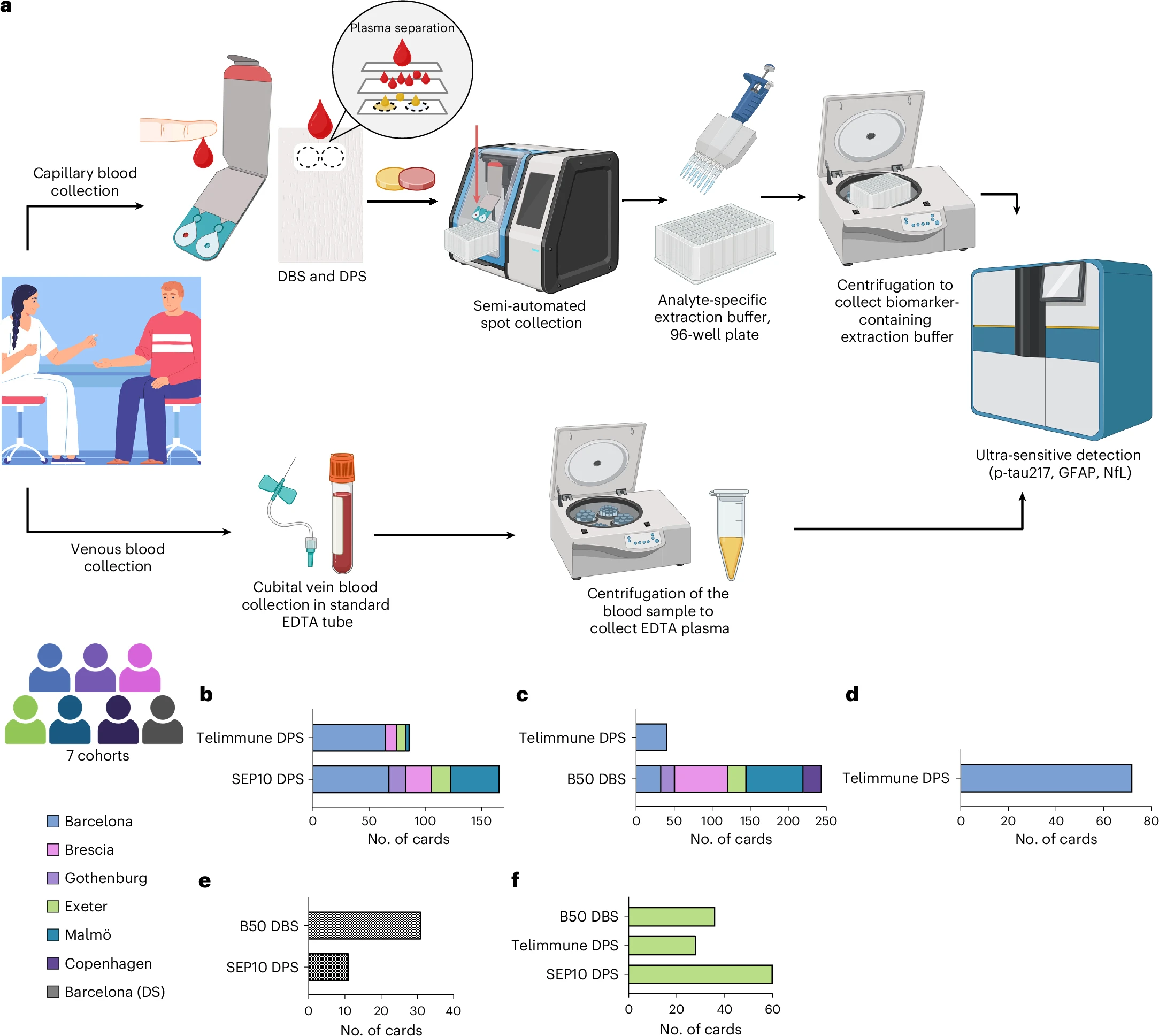
The Simoa Dry Blood Extraction Kit workflow is supported by evidence from the DROP-AD study, published in Nature Medicine, demonstrating that capillary dried blood samples can be used to reliably quantify key Alzheimer’s disease biomarkers.
The multi-centre European study showed strong concordance between capillary dried blood and conventional venous plasma measurements for:
p-Tau217 – strong correlation across multiple sites
GFAP and NfL – reliable quantification with high plasma concordance
The approach demonstrated good diagnostic accuracy for identifying CSF-confirmed Alzheimer’s pathology and high reproducibility with self-collected samples, supporting decentralized and remote study designs.
Importantly, the study confirms the feasibility of this approach in high-risk and underrepresented populations, including individuals with Down syndrome, and eliminates the need for venipuncture, centrifugation, and cold-chain logistics.
This workflow is intended for research use only and is not designed for clinical diagnosis or clinical decision-making.
The Simoa Dry Blood Extraction Kit is designed to support high-sensitivity biomarker analysis in settings where traditional venous sampling and cold-chain logistics are limiting.
For academic and translational neuroscience teams conducting large cohort or longitudinal studies, the kit provides a standardized method for extracting biomarkers from small-volume, remotely collected samples, reducing pre-analytical variability and improving inter-site comparability.
In pharmaceutical and biotechnology research, the workflow supports early-phase and decentralized study designs where sample volume is limited but analytical sensitivity is critical.
For public health and global research settings, the kit enables reliable laboratory-grade biomarker analysis from capillary dried blood samples, supporting studies in low-resource or geographically distributed populations.
The Simoa Dry Blood Extraction Kit is intended for the extraction of analytes from capillary-derived dried blood and plasma collection devices to support detection of low-abundance biomarkers using Simoa® assay kits.
For research use only.
Each kit includes Quanterix extraction buffer and two precipitation plates.

Quanterix Business Manager
As the official distributor of Quanterix in Australia and New Zealand, Decode Science is providing local access to Simoa® platforms, assays, and workflow solutions with region-based technical and application support.
This site uses cookies. By continuing to browse the site, you are agreeing to our use of cookies.
OKLearn moreWe may request cookies to be set on your device. We use cookies to let us know when you visit our websites, how you interact with us, to enrich your user experience, and to customize your relationship with our website.
Click on the different category headings to find out more. You can also change some of your preferences. Note that blocking some types of cookies may impact your experience on our websites and the services we are able to offer.
These cookies are strictly necessary to provide you with services available through our website and to use some of its features.
Because these cookies are strictly necessary to deliver the website, refusing them will have impact how our site functions. You always can block or delete cookies by changing your browser settings and force blocking all cookies on this website. But this will always prompt you to accept/refuse cookies when revisiting our site.
We fully respect if you want to refuse cookies but to avoid asking you again and again kindly allow us to store a cookie for that. You are free to opt out any time or opt in for other cookies to get a better experience. If you refuse cookies we will remove all set cookies in our domain.
We provide you with a list of stored cookies on your computer in our domain so you can check what we stored. Due to security reasons we are not able to show or modify cookies from other domains. You can check these in your browser security settings.
These cookies collect information that is used either in aggregate form to help us understand how our website is being used or how effective our marketing campaigns are, or to help us customize our website and application for you in order to enhance your experience.
If you do not want that we track your visit to our site you can disable tracking in your browser here:
We also use different external services like Google Webfonts, Google Maps, and external Video providers. Since these providers may collect personal data like your IP address we allow you to block them here. Please be aware that this might heavily reduce the functionality and appearance of our site. Changes will take effect once you reload the page.
Google Webfont Settings:
Google Map Settings:
Google reCaptcha Settings:
Vimeo and Youtube video embeds:
The following cookies are also needed - You can choose if you want to allow them:
Simply add your details below and get quick access to the brochure.
Simply add your details below and get quick access to the brochure.
Simply add your details below and get quick access to the brochure.
Simply add your details below and get quick access to the brochure.